The first innovation in frown line treatments in 20 years.
See how our peptide formulation is different.
The science of our peptide formation.
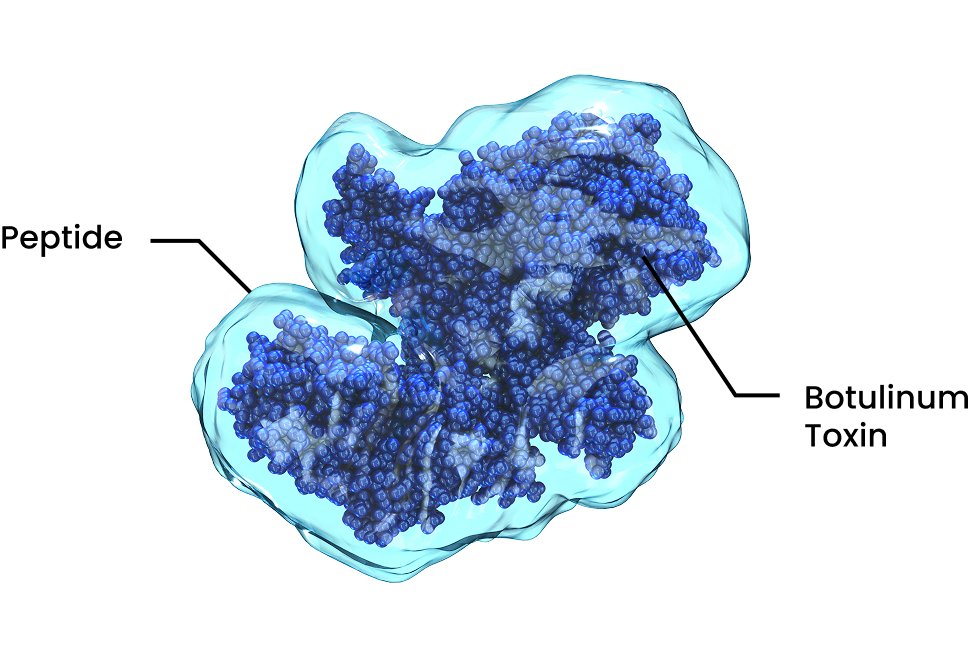
Our formulation uses a novel peptide that forms a strong, magnet-like bond with the nerve cells, helping to deliver the active ingredient efficiently and remain available to work over time. Watch the video to learn more.
It’s also the first formulation to stabilize the active ingredient without the use of human blood products or animal byproducts.
Fast, long-lasting, and radiant-looking results—delivered by science you can feel good about.
*In clinical trial diaries, patients reported seeing a measurable improvement within 2 days of treatment. 74% achieved a ≥ 2-grade improvement at week 4 per both physician's and patient’s assessment. More than 50% had no or minor frown lines until 6 months after treatment per both physician's and patient's assessments. In an open label study (N=27), 96% of patients agreed their skin looked smooth in the glabella (exploratory endpoint).
See our peptide formulation in action.
See real results.
Seeing is believing. Explore real, radiant-looking results that speak for themselves.*
*In clinical trial diaries, patients reported seeing a measurable improvement within 2 days of treatment. 74% achieved a ≥ 2-grade improvement at week 4 per both physician's and patient’s assessment. More than 50% had no or minor frown lines until 6 months after treatment per both physician's and patient's assessments.
See moreFind DAXXIFY® near you.
Ready to try the first frown line treatment with a peptide formulation? Find a licensed provider and book today.
locate a providerPatients prefer DAXXIFY®.
of patients preferred DAXXIFY® over their previous treatment within two weeks.*
of patients were satisfied with their treatment at week 4.†
*Based on a survey of 149 patients at week 2.
†96% of patients reported they were satisfied
on a 7-point scale when evaluated at 4 weeks in clinical studies.
Why providers pick
DAXXIFY®
"The reaction that my patients have to the fact that [the appearance of] their skin texture is improved and the fact that they're getting a long-lasting product has made it an elevated experience for my patients."*
*In an open label study (N=27), 96% of patients agreed their skin looked smooth in the glabella (exploratory endpoint). More than 50% had no or minor frown lines until 6 months after treatment per both physician's and patient's assessments.

U.S.A.-made, clinically proven.
DAXXIFY® is the only frown line treatment manufactured in the U.S.A.
Ready to take the next step?
Find a provider.
FIND DAXXIFY®
See fast-acting results.
See results